News
Gene and Cell Therapy Event Insights
The Gene and Cell community can get excited for this year's edition 2nd Annual Gene and Cell Therapy: Quality Developments to Commercialization Summit. This business conference attracts professionals like Chief Executives, Vice Presidents, Directors, Heads, Leaders, and Managers specializing in Advanced Therapy Production, Analytical, Biotherapeutics, Cell, Engineering, Cellular Immunotherapy, Clinical Development, Gene Therapy, GTMPs (Gene Therapy Medicinal Products), Health Economics, Immuno-Oncology, Immunology Chemistry, Legal/IP, Market Access, Oncology, Pricing, Quality Control, Regenerative Medicine, Safety, Stem Cell, Tissue Therapy, ATMP (Advanced Therapy Medicinal Product), Bioprocessing, CAR-T, Cell Therapy, Cellular Therapy, CMC, GMP, CBMPs (Cell-Based Medicinal Products), Immune deficiencies, Immunology, Immunotherapy, Manufacturing, Medical, Precision Medicine, Process Development, R&D, Regulatory, Sales, Supply Chain.
2020 Key Objectives
Key objectives of the upcoming 2nd Annual Gene and Cell Therapy: Quality Developments to Commercialization Summit 2020 include:
- Optimized cell and gene therapies process development, manufacturing, cost and quality efficiency towards successful commercialization
- CMC technical and regulatory compliance to empower cell and gene therapy products development roadmap
- Emerging science and technologies to enhance cell and gene therapies development
- Robust supply chain design for precision medicine therapies
- Advanced analytical approaches to improve quality control and real-time release
Our company has garnered vast experience over the years, creating quality conferences and summits that provide our participants with innovative ideas. In 2020, to keep up with the evolving digital world, we have also introduced a series of Live Events.
Our Gene and Cell Therapy: Regulatory CMC, Quality Development, and Manufacturing Live Event had been streaming on the 23rd of June 2020. The recording of the live event is available on our website. By purchasing this recording, you will get access to 7 insightful presentations from speakers coming from respectable companies offering you a chance to learn more about recent strategies, perspectives, and expectations in the field. Get access to the recording by contacting us here.
Gene and Cell Therapy: Quality Developments to Commercialization Summit took place on 5th – 6th June 2019 in Berlin, Germany. This is an annual business meeting dedicated exclusively to furthering knowledge and exploring the Gene and Cell Therapy process development. This summit is traditionally held with the partnership with the ProJect Pharmaceutics GmbH, Beckman Coulter, Inc., and other partners. It brings together key decision-makers from all over the world and encourages timely discussions on gene and cell subjects.
Last year we were delighted to welcome original speakers, enthusiastic delegates, and respectable sponsors from the industry. Let's have a look at what exciting presentations were presented to our curious audience.
---------------------------------------------------------------------------------------------------------------------------
The following text was inspired by the speakers presenting at Gene and Cell Therapy: Quality Developments to Commercialization Summit 2019. Media materials contain only public information and have been prepared solely for Vonlanthen Group Event educational purposes of contributing to the understanding of the Gene and Cell Therapy. These materials reflect only the author's personal views and may not be the views of his employer.
---------------------------------------------------------------------------------------------------------------------------
1. CGT strategy: Break Through Innovation Therapies in Order to Treat More Common Diseases by Dr. RITA MAJITHIYA
Dr. Rita Majithiya is a CMC drug product lead for cell and gene therapy projects at GSK with significant experience of drug development pathway and regulatory requirements for drug products towards commercialization of assets.
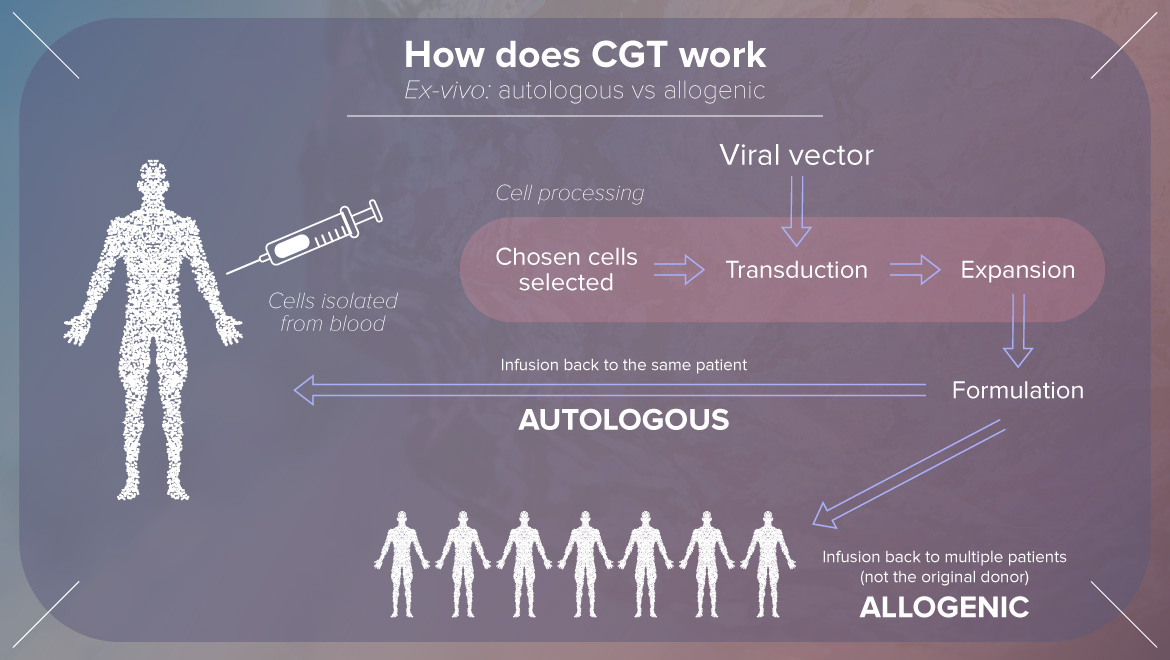
Rita focused on formulation development considerations for autologous cell and gene therapy products to enable robust vein to vein delivery. She explained how does CGT work and what is the GSK’s CGT strategy and many more interesting topics.
2. Commitment to a Cure. Beyond Knock-Out: Designing Smarter, Safer, and More Efficient Allogenic UCART Product Candidates for Patients by STEPHEN REYNIER
As chief regulatory and compliance officer at Cellectis, Stephan is in charge of ensuring a speedy and successful development of the UCART product family by establishing close interactions with regulatory agencies such as EMA and FDA, while securing compliance to applicable regulations, regulatory guidelines, and quality assurance standards.

Stephen centered his presentation around “forward-looking” statements that are based on his company management’s current expectations and assumptions and information currently available to management. He introduced known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
3. Strategies and Advances in AAV Vector Characterization by CHRISTINE LE BEC
Head of the CMC Analytical Department, responsible for the analytical activities in the characterization and release testing of gene therapy products at early-stage development, stability studies, and interface with CMO for method transfer and validation, analytical/QC testing. Christine shared valuable information about the best tools for vector genome titration, the potential impact of empty particles on AAV product quality, and empty/full AAV particles quantitation. 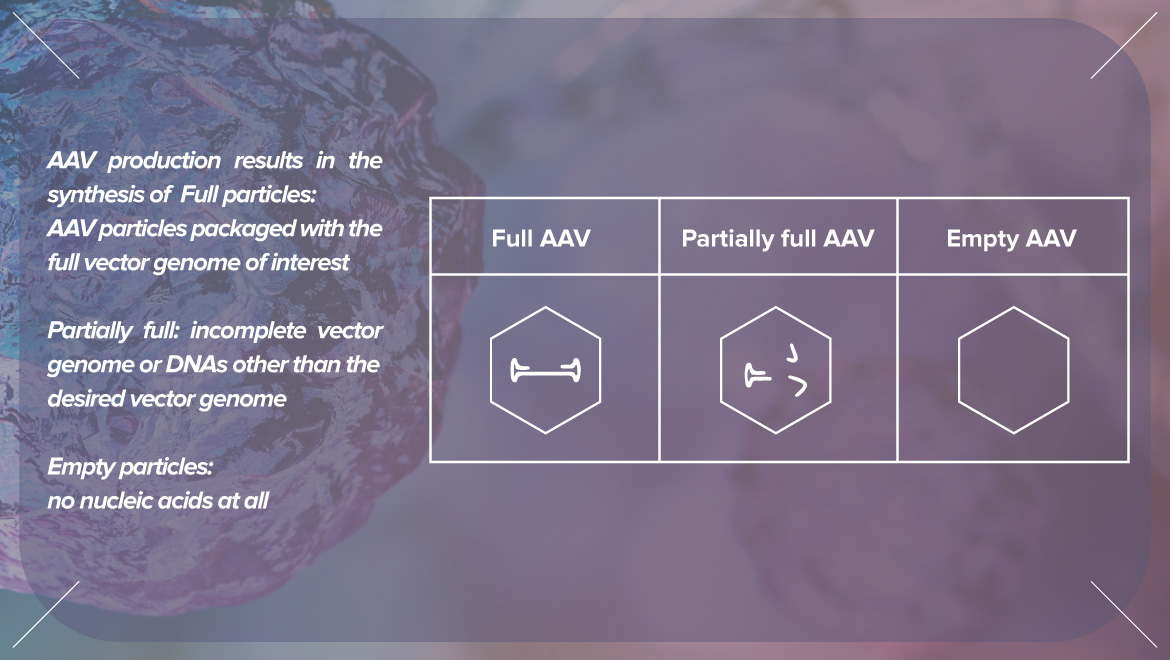
4. Large scale production of LV and RV for transduction of T- and Cd34 + cells Dr. MANUELA COTA
Manuela Cota is an upstream process development supervisor in MolMed. She is involved in the process development and industrialization of large-scale production of lentiviral and retroviral vectors in compliance with GMP requirements.
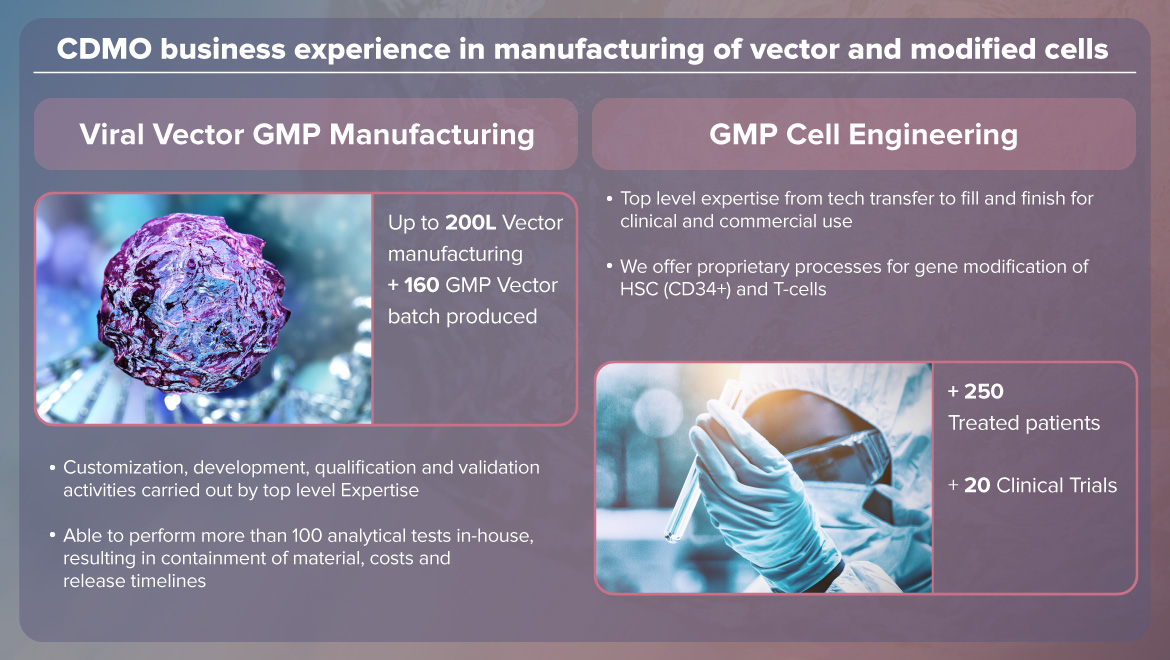
"MolMed is implementing versatile close systems to produce high-quality vectors for clinical trials and market from 48L to 200L based on 293T adherent cell line. iCellis system allows the development of fully cGMP compliant manufacturing processes for RV and LV vectors.
Vectors produced in iCellis systems:
- are qualitatively comparable to CF clinical batches
- batch size is cost-effective in terms of the number of patients treated versus the cost of production and QC
- high reproducibility of clinical DP batches iCellis500 engineering runs are planned in GMP environment to conclude tech transfer and Process"
5. Process Development and Manufacturing Strategies for AAV Products by MARIAN BENDIK
Marian is Site Lead in Orth for Gene Therapy Center Austria at Takeda. He holds accountability for gene therapy manufacturing and acts as site lead in Orth for Gene Therapy Center Austria at Takeda.
In his presentation on Orphan Drugs, he introduced the topic Fast to Clinic vs. Fast to Market?

6. The regulatory challenges and practicalities of working with genetically-modified organisms by Dr. KATHRYN PARSLEY
Kathryn Parsley is a biological science graduate with a master of philosophy in microbiology/immunology and a PhD in molecular immunology/gene therapy. In her presentation, she introduced GM based IP’s, objective of the risk assessment, process for its preparation and documentation, and the reason for their regulation and summarized the challenges for the future.

"Domestication of plant and animals has occurred since about 12,000 BC by selective breeding. Plants and animals with the most desirable characteristics are chosen for breeding the next generations. Selective breeding depends on genetic variation within a population. Genetic engineering involves the intentional introduction of genes from different species. Scientists are able to manipulate, remove, and add genes to a variety of different organisms to induce a range of different traits. Such organisms are called GMOs."
Genetic Modification, GMOs
"Genetic modification involves the mutation, insertion, or deletion of genes. Inserted genes usually come from a different species in the form of horizontal gene transfer. In nature, this can occur when exogenous DNA penetrates the cell membrane for any reason. To do this artificially may require:
- attaching the genes to a virus
- physically inserting the extra DNA into the nucleus of the intended host with a very small syringe with electroporation (electric pulse)
- exploiting natural forms of gene transfer, such as the use of the ability of Agrobacterium to transfer genetic material to plants, or the ability of lentivirus to transfer genes to animal cells"
7. Preclinical safety assessment of AAV-mediated gene therapy from a pathologist perspective by Dr. BASEL ASSAF at Pfizer
Dr. Basel Assaf is a versatile board-certified pathologist-scientist with well-balanced and integrated advanced training in pathology, virology, immunology, gene therapy, and FDA regulatory review process. He has touched about many interesting topics in his presentation like Introduction to Gene Therapy (GTx), Adeno-Associated Virus Basics, or Design of Preclinical Safety Program.

8. CMC strategies for commercialization of gene therapy by CATHERINE CANCIAN
Catherine Cancian is responsible for the management of the pharmaceutical development programs from preclinical to phase III clinical studies and commercialization at GenSight.

GenSight targets 3 areas of unmet needs: LHON, RP & DRY AMD:
- "Genetic disorders and aging are responsible for retinal degenerative diseases that lead to blindness.
- Therapeutic gene is packaged in a virus vector (AAV) AAV is injected into the eye (intravitreal or subretinal).
- AAV vector expresses a therapeutic protein into the Retinal Ganglion Cells. It enables the retina to regain lost function."
9. Nonclinical, Clinical, and CMC Analytical Activities by Dr. Paul Byrne, at Covance
Paul focusses on the analytical support required for cell and gene therapy molecules within the preclinical, CMC, and clinical testing areas. During his speech he talked about the Nonclinical in life consideration, Analytical design and validation strategies for Bioanalytical, Biodistribution and clinical studies, CMC considerations and Phase appropriate validations for CMC assays.
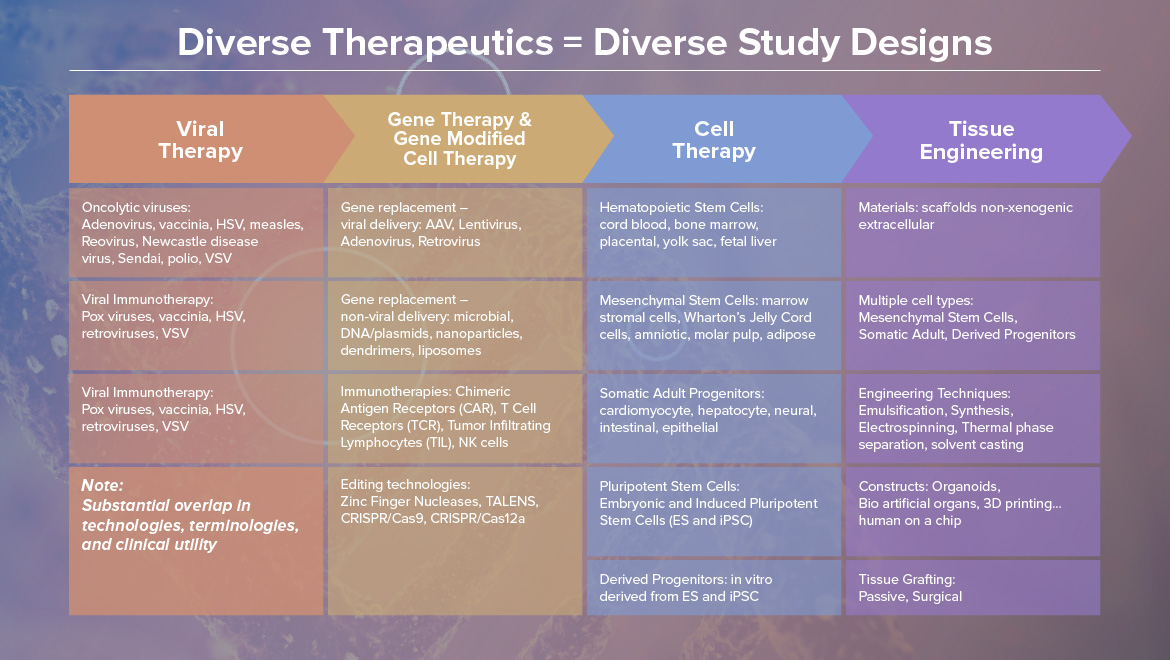
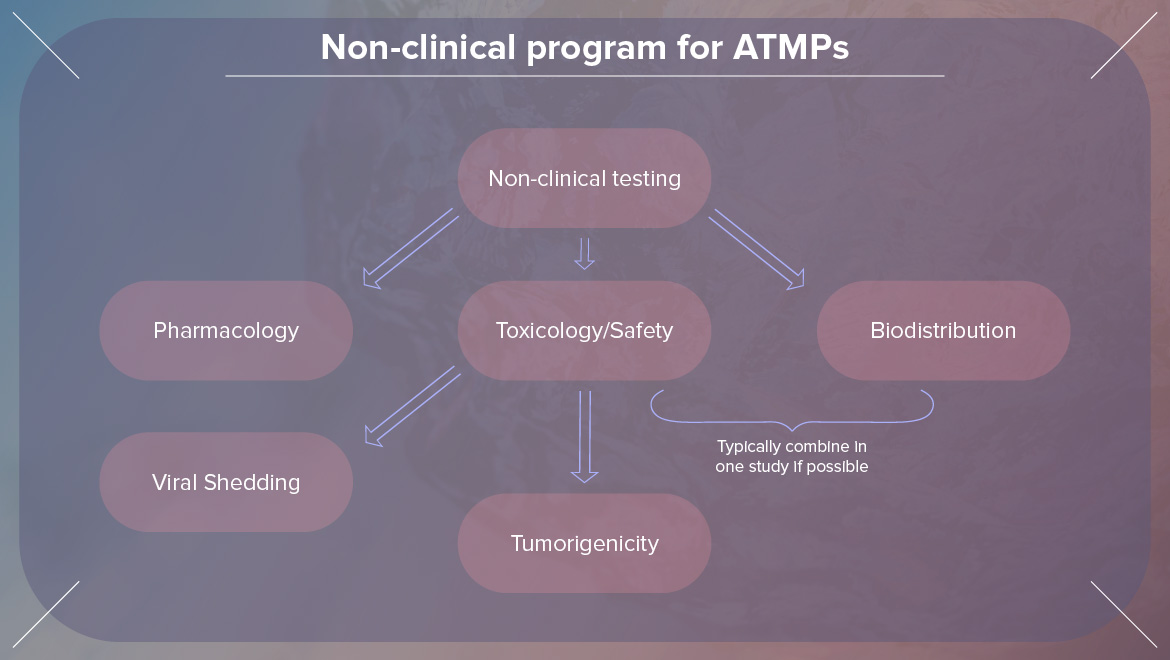
We are excited to meet the next line-up of speakers this Autumn in Germany. Previous attendees appreciated networking opportunities as it is a vital part of our events and gained so many new insights to apply in their works and companies. If you are interested in sharing your experience with other industry professionals, don't hesitate to contact us.
We will be delighted to have you as an attendee at ou Gene and Cell Therapy Summit 2020. If you enjoyed this article, feel free to share it with your peers.

